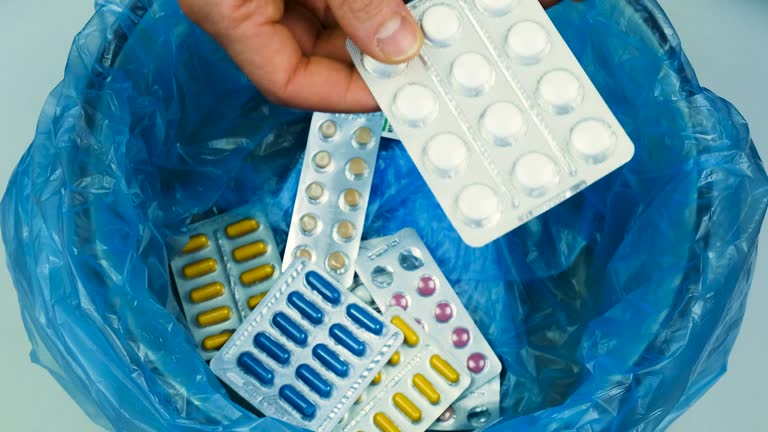
Throwing pharmaceutical waste, such as hazardous drugs, sharps, controlled substances, and expired medications, in the regular trash or flushing it down the drain may seem harmless, but the consequences can be severe. They can end up in the water lines, contaminating the drinking water or posing serious health risks to healthcare workers, waste handlers, and the general public.
That is why healthcare facilities and pharmacies need to handle waste correctly. Failure to comply with these regulations can result in hefty fines, legal liabilities, and reputational damage.
But how do you perform safe and compliant pharmaceutical waste disposal? Well, it starts with identifying the different types of waste and following the correct disposal procedures with color-coded containers, all of which you will learn in this blog post! By this end, you will have insights to support ethical and efficient pharmaceutical waste management.
Types of Pharmaceutical Waste
Not all waste is created equal. Pharmaceutical waste comes in various forms, and understanding these categories is integral to ensuring proper disposal. Below are the most common types:
Manufacturing Waste
This type of waste stems from the production of pharmaceutical products. During manufacturing, byproducts, raw materials, and even faulty products may need to be disposed of to maintain safety and efficiency in operations. Examples include:
- Residual Chemicals: After the production process, excess active pharmaceutical ingredients (APIs) or other chemicals may remain, requiring proper disposal.
- Unused Raw Materials: Some raw materials, like excipients or binders, may go unused due to batch size adjustments or contamination.
- Off-Specification Products: If a drug batch does not meet quality standards, it cannot be distributed and must be disposed of to prevent unsafe medication from reaching consumers.
Clinical Trial Waste
Clinical trials generate medical waste from testing new treatments, including unused investigational drugs, expired medications, and contaminated lab materials. Examples Include:
- Unused Investigational Drugs: Experimental medications that are no longer viable for trials must be disposed of safely.
- Expired Trial Samples: Outdated formulations used in studies require specialized destruction to prevent unauthorized use.
- Contaminated PPE and Lab Supplies: Gloves, syringes, and vials exposed to trial substances must be handled as hazardous waste.
Expired and Recalled Products
Expired medication disposal is one of the most common types of pharmaceutical waste management encountered by pharmacists and healthcare providers. Every pharmaceutical product comes with an expiration date, and for legal compliance, unused drugs past their expiration must be disposed of in specific containers. Recalled products follow the same disposal guidelines to avoid environmental contamination or inadvertent reuse.
Chemical and Solvent Waste
Pharmaceutical processes often involve chemicals and solvents, many of which may be toxic, flammable, or corrosive. Examples include:
- Solvent-Based Drug Formulation: The creation of liquid-based medications may leave behind hazardous solvent residues.
- Cleaning Agents: Strong chemicals used to clean manufacturing equipment contribute to hazardous waste.
- Chemical Reactions: Various chemical reactions in the synthesis of drugs may generate byproducts requiring proper disposal.
Packaging Waste with Residual Pharmaceuticals
Packaging materials that previously held pharmaceuticals may still contain traces of active ingredients, requiring careful disposal. Here are a few examples:
- Blister Packs: These may have leftover drug particles or residue.
- Glass Vials and IV Bags: Residual liquid medications can remain in the packaging.
- Syringes and Ampoules: These often contain small amounts of injectable drugs that must be disposed of properly.
Segregation in Waste Containers
Segregation involves sorting pharmaceutical waste into different categories based on its type, composition, and disposal requirements.
- Key Steps for Effective Segregation:
- Identify Waste Categories: Separate waste into categories such as hazardous, non-hazardous, sharps, chemical waste, and expired medications.
- Use Color-Coded Containers: Assign specific waste bins for each category to prevent cross-contamination. For example:
- Black containers: Hazardous pharmaceutical waste
- Blue containers: Non-hazardous pharmaceutical waste
- Yellow containers: Infectious or biohazardous waste
- Red containers: Sharps (e.g., needles, syringes)
- Regular Monitoring: Avoid overfilling, replace bins when needed, and maintain a clean disposal area.
- Secure Placement: Position bins in controlled, accessible areas to prevent unauthorized access.
- Educate Employees: Train staff on waste segregation procedures and proper bin usage.
- Label Clearly: Use bold signage with descriptions and pictograms to guide proper disposal.
Pro Tip: To prevent leaks and contamination, the containers must be made of high-density polyethylene (HDPE), stainless steel, or rigid plastic.
Proper Disposal Methods
After segregation comes disposal. Here are the methods to dispose of pharmaceutical waste properly:
Incineration
Incineration is the process of burning hazardous pharmaceutical waste at high temperatures (typically above 1,000°C) in specialized facilities to completely neutralize harmful substances.
How to Implement:
- Use Licensed Incineration Facilities: Work with authorized medical waste incineration plants that comply with environmental regulations.
- Pre-Treat Some Waste If Needed: Some pharmaceutical waste may require preprocessing before incineration (e.g., crushing or encapsulation).
- Monitor Air Emissions Compliance: Confirm that incineration facilities meet air pollution control standards to minimize environmental impact.
- Document Disposal: Maintain records of incinerated waste to demonstrate regulatory compliance.
Secure Landfill Disposal
Some pharmaceutical waste can be disposed of in specially designed landfills that prevent leaching and contamination of groundwater. These landfills use protective liners and containment systems to isolate hazardous substances.
How to Implement:
- Confirm Acceptable Waste Types: Only non-hazardous pharmaceutical waste and specific hazardous materials (approved by local regulations) should go to secure landfills.
- Work with Certified Landfills: Partner with waste management companies that operate secure landfills meeting environmental safety standards.
- Use Proper Packaging: Ensure waste is stored in sealed, non-leaking containers before disposal.
- Monitor and Report Compliance: Keep records of landfill disposal activities for regulatory audits.
Reverse Distribution Programs
Reverse distribution programs allow expired, recalled, or unused pharmaceutical products to be collected and returned to authorized handlers for proper disposal or potential credit recovery.
How to Implement:
- Partner with Licensed Reverse Distributors: Work with authorized companies that specialize in medical waste pickup returns and waste management.
- Develop a Collection System: Establish procedures for gathering expired and recalled medications from pharmacies, hospitals, and manufacturers.
- Secure Compliance: Follow federal and local laws governing pharmaceutical returns and disposal.
- Prevent Diversion: Secure all expired and recalled medications in tamper-proof containers to prevent unauthorized access.
- Track Waste Movements: Maintain records of all pharmaceutical products sent through reverse distribution for audit purposes.
Pharmaceutical Waste Disposal Compliance and Regulations
Pharmaceutical waste disposal is governed by stringent regulations to safeguard human health and the environment. Non-compliance can result in hefty fines, reputational damage, or, worse, harm to public well-being.
Legal Compliance for Pharmaceutical Waste
Federal and state bodies such as the Environmental Protection Agency (EPA) and the Drug Enforcement Administration (DEA) in the United States enforce compliance requirements for pharmaceutical waste. Key rules include:
- Resource Conservation and Recovery Act (RCRA): Governs hazardous waste disposal.
- DEA Regulations: Define procedures for controlled substances.
- USP <800>: A guideline for managing hazardous drugs in healthcare environments.
In a Nutshell
Now that you know how to dispose of pharmaceutical waste using compliant waste containers, the final step is finding a trusted and reliable waste management partner. At Secure Waste, we provide reliable, compliant, and eco-friendly pharmaceutical waste disposal solutions designated to your facility’s needs. With expertise in RCRA, DEA, and EPA regulations, we provide customized waste management plans, including secure collection and transport and sustainable disposal practices.
Contact us today for a FREE Waste Assessment, or request a quote online!

Expert Medical Waste Management: With over 25 years of industry experience, Secure Waste is a trusted local leader in hazardous and biohazardous waste disposal across Maryland, Virginia, and Washington, D.C. Specializing in medical waste management, sharps needle disposal, and biohazard waste removal, the company ensures full compliance with federal, state, and local regulations while prioritizing environmental sustainability.
The company also offers additional services, including secure document shredding and sharps container sales, providing comprehensive solutions for healthcare facilities and businesses. Our cost-effective services help clients maintain regulatory compliance without unexpected costs.
With a commitment to customer satisfaction, Secure Waste offers tailored waste management plans that align with industry best practices. Their team of experts provides reliable, timely, and compliant services, making them the preferred choice for medical waste disposal. For a free waste quote or more information, visit www.securewaste.net